News
Here's The Next Major SCOTUS Abortion Case
Here's the genius behind the wave of abortion restrictions that have swept the nation since the Republican victories in 2010: the language of the new laws enables them to sound reasonable and safety-based, and authored out of concern for women's health — when in actuality they limit our access to safe healthcare.
And as Slate points out, the 2011 Oklahoma law that limits doctors' ability to prescribe the pills that help terminate early pregnancies is no exception.
A challenge to the law promises to be the next big case the Supreme Court hears about abortion.
The New York Times Opinionator blog explains that the court's decision to take up the case is a momentous one, because it signifies that the justices saw the Oklahoma case as a potential platform to make a major statement about abortion and its regulation.
Specifically, medical abortion.
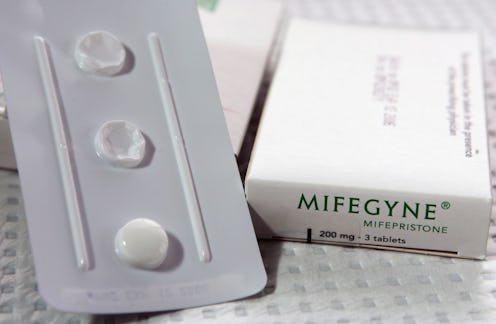
"Medical abortion" is most effective in the first six or seven weeks of pregnancy and doesn't work at all after nine weeks. The procedure is simply the consumption of two pills: mifepristone to block the hormone progesterone, without which a pregnancy can't proceed, and misoprostol, which causes the uterus to contract and expel the early pregnancy when taken two days later. In a lot of states, women can take the second pill at home. Opinionator's Linda Greenhouse calls the procedure "the ultimate in women's reproductive empowerment and personal privacy."
No wonder anti-choice activists are so distressed by it.
Like much other anti-choice legislation, the Oklahoma law is cleverly designed to appear as a bill championing healthcare safety standards. The law was actually authored in Chicago by the influential anti-abortion group Americans United for Life, and included the predictably titled "Abortion-Inducing Drugs Safety Act," which makes it a crime for doctors to deviate from the dosage and instructions published by the Food and Drug Administration when it approved the drug in 2000.
What's so wrong with that? Quite a lot. The FDA's instructions for taking the medication were, by my middle school level math skills, 13 years old. What this means is that medical opinion about how to take the drug has significantly evolved in the last decade plus — which is typical after a new medication enters widespread use.
For example, instead of 600 milligrams of Mifeprex, doctors now use only 200. The original FDA instructions said the medication should be used only up to 49 days of pregnancy, but doctors now know the procedure is safe and effective for up to 62 days. Furthermore, doctors now often give the patient the second drug to be taken in the privacy of her own home, despite the fact that the FDA mandates a second office visit.
So you see where the law starts to be less about advocating for women's health and more about limiting it. What's more is that the 600-milligram dose is now considered bad medicine, and many doctors would wholly refuse the procedure rather than follow the obsolete guideline.
The state trial judge Donald L. Worthington's review backed up this finding. Worthington concluded that requiring doctors to use the higher dose was “so completely at odds with the standard that governs the practice of medicine that it can serve no purpose other than to prevent women from obtaining abortions and to punish and discriminate against those women who do.”
The Oklahoma Supreme Court affirmed the decision last December, but because the state high court's unanimous three-paragraph opinion (which offered no analysis) declared that "this matter is controlled by the United States Supreme Court decision in Planned Parenthood v. Casey," the law remained.
Keep your eyes out for the likely forthcoming and monumental case.